Growth and morphology metal-organic films
Metal-organic films deposited by the Langmuir-Blodgett (LB) technique have been the subject of intense research due to their ease of preparation, potential applications in various fields, and availability of sophisticated structural characterization techniques, such as atomic force microscopy (AFM) and X-ray & neutron reflectometry. X-ray scattering studies had shown 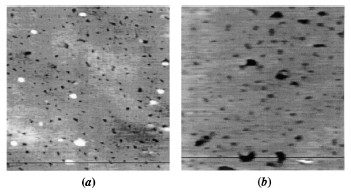 that the interfacial correlation of these films can vary from self-affine fractal, observed in diverse physical systems, to long range logarithmic, characteristic of capillary waves on liquid surfaces. It is expected that systematic studies of these interfacial morphology can provide us a clue to the growth mechanism of LB films, especially because a lot of theoretical and simulation studies have been performed to link the evolution of the interfacial morphology with the possible growth mechanism of thin films. that the interfacial correlation of these films can vary from self-affine fractal, observed in diverse physical systems, to long range logarithmic, characteristic of capillary waves on liquid surfaces. It is expected that systematic studies of these interfacial morphology can provide us a clue to the growth mechanism of LB films, especially because a lot of theoretical and simulation studies have been performed to link the evolution of the interfacial morphology with the possible growth mechanism of thin films.
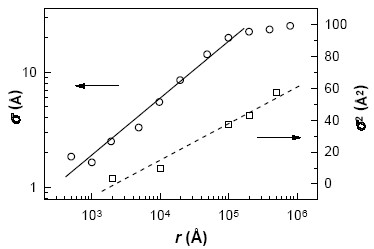 To understand this growth mechanism of fractal surface morphology seen in many diverse physical systems of general interest, we have performed X-ray scattering and AFM studies on Cd-arachidate LB films exhibiting self-affine and logarithmic in-plane correlation at the interfaces. Using linear stochastic theory for interface evolution, it is proposed that a 1D deposition followed by a 2D desorption process is the growth mechanism of LB films. X-ray and AFM measurements confirm the crossover between these
two growth regimes [published in Phys. Rev. Lett. 82, 4675 (1999)].
To understand this growth mechanism of fractal surface morphology seen in many diverse physical systems of general interest, we have performed X-ray scattering and AFM studies on Cd-arachidate LB films exhibiting self-affine and logarithmic in-plane correlation at the interfaces. Using linear stochastic theory for interface evolution, it is proposed that a 1D deposition followed by a 2D desorption process is the growth mechanism of LB films. X-ray and AFM measurements confirm the crossover between these
two growth regimes [published in Phys. Rev. Lett. 82, 4675 (1999)].
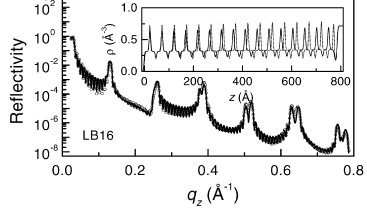 Metal-organic multilayers of Mn-stearate LB films on hydrophobic Si
substrate were studied by grazing incidence X-ray scattering techniques. Grazing incidence X-ray diffraction measurements show distorted hexagonal in-plane structure of the molecules. Refectivity measurements show that the LB films consist of two types of blocks having slightly different bilayer separation, but well arranged. The difference in bilayer separation suggests that the modecules in one block are tilted with respect to the other. The reasons for which these LB films present such imperfection are discussed [published in Physica B 283, 45 (2000)].
Metal-organic multilayers of Mn-stearate LB films on hydrophobic Si
substrate were studied by grazing incidence X-ray scattering techniques. Grazing incidence X-ray diffraction measurements show distorted hexagonal in-plane structure of the molecules. Refectivity measurements show that the LB films consist of two types of blocks having slightly different bilayer separation, but well arranged. The difference in bilayer separation suggests that the modecules in one block are tilted with respect to the other. The reasons for which these LB films present such imperfection are discussed [published in Physica B 283, 45 (2000)].
Langmuir monolayers, i.e. monomolecular layers of amphiphilies at the air-water interface, are fascinating examples of 2-dimensional systems. These compressed monolayers can be transferred on to some solid substrate to form LB films. Their physico-chimical properties can be modified very easily by changing temperature, pressure and pH of the aqueous subphase and also by dissolving different ions in the subphase. Some of the most ordered LB films such as Cd-arachidate are normally deposited from
arachidic acid monolayers on water containing cadmium ions at comparatively low pH, which has definite headgroup-metal interaction in the Langmuir monolayer. It has been observed that by changing the pH it is possible to change the interaction and thus to increase the metal content in the head-group. In this project, out research plan is to utilize this idea to grow LB films containing high amount of metals in the head-group.
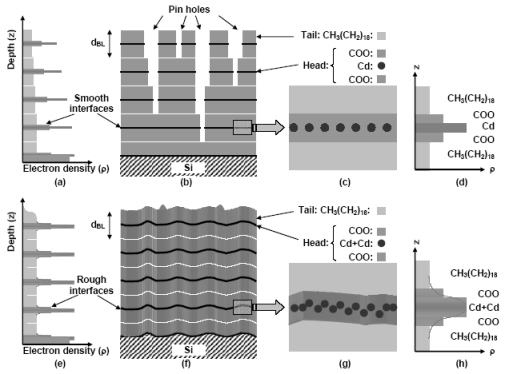 Cd-arachidate LB films, deposited at 'normal' (~6.5) and 'high' (~9.0) subphase pH on hydrophilic Si(001) have been studied by AFM and X-ray reflectivity techniques. Surface topography and electron density profiles (EDP) reveal absence of pinhole type defects in the 'high pH' LB film, but presence of ridges aligned roughly parallel to the direction of deposition. EDP of the films indicate nearly one extra Cd-ion per headgroup for 'high pH' films, suggesting clearly for the first time that more metal ions can be incorporated in LB films through subphase pH variation. Fourier transform infrared (FTIR) spectra indicate formation of bidentate bridging type headgroups consistent with one more metal ion per headgroup [published in
Chem. Phys. Lett.
405, 282 (2005)]. Cd-arachidate LB films, deposited at 'normal' (~6.5) and 'high' (~9.0) subphase pH on hydrophilic Si(001) have been studied by AFM and X-ray reflectivity techniques. Surface topography and electron density profiles (EDP) reveal absence of pinhole type defects in the 'high pH' LB film, but presence of ridges aligned roughly parallel to the direction of deposition. EDP of the films indicate nearly one extra Cd-ion per headgroup for 'high pH' films, suggesting clearly for the first time that more metal ions can be incorporated in LB films through subphase pH variation. Fourier transform infrared (FTIR) spectra indicate formation of bidentate bridging type headgroups consistent with one more metal ion per headgroup [published in
Chem. Phys. Lett.
405, 282 (2005)].
Langmuir monolayer of stearic acid on pure water and in presence of certain divalent metal ions like Cd and Pb at pH ~ 6.5 of the subphase water collapses at constant area, while for other divalent ions like Mg, Co, Zn and Mn at same subphase pH the monolayer collapses nearly at constant pressure. Films of stearic acid with Cd, Pb, Mn and Co in the subphase (at pH ~ 6.5) have been transferred onto hydrophilic Si(001) using a horizontal deposition technique, just after and long after collapse. EDP obtained from X-ray reflectivity analysis show that a three-molecular layer structure starts to form just after constant area collapse where the lowest molecular layer, in contact with the substrate, molecules in asymmetric configuration, i.e., both hydrocarbon tails on the same side of the metal-bearing headgroup that touches the substrate, while the molecules above the first layer are in symmetric conformation of tails with respect to the headgroups. Further along collapse, when the surface pressure starts to rise again with decrease in area, more layers with molecules in the symmetric configuration are added but the coverage is poor. On the other hand, only bimolecular layers form after constant pressure collapse with the lower and upper layers having molecules in asymmetric and symmetric configurations respectively and the upper molecular layer density increases with compression of monolayer after collapse. A 'Ries mechanism' for constant area collapse and a 'folding and sliding mechanism' for constant pressure collapse have been proposed [published in Langmuir 21, 5894 (2005)].
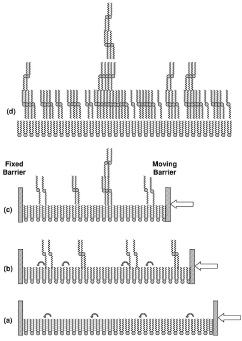 Langmuir monolayers of stearic acid on Co-ions in the aqueous subphase have been deposited at different stages of constant pressure collapse, on hydrophilic Si(001) using a modified version of the inverse Langmuir-Schaefer method of horizontal deposition. The EDPs along the depth of the deposited films, extracted from the x-ray reflectivity data, show that a monolayer to bi-molecular layer transformation takes place after collapse. The molecules in the lower monolayer have asymmetric configurations with headgroups touching water and tails in air, whereas molecules in the upper layer are in symmetric configurations with tails on both sides of the heads. AFM images of the deposited films after collapse, however, show nearly circular islands of height more than that of the bimolecular layer observed in the EDP. As pressure increases, ridges are seen to coexist with these islands. Although the coverage of such islands and ridges is low, they play an important role in determining the growth mode. The growth of the wetting and island layers, taken together, has a striking similarity with the Stranski-Krastanow mode, observed usually for heteroepitaxial growth [published in Phys. Rev. E 73, 051608 (2006)].
Langmuir monolayers of stearic acid on Co-ions in the aqueous subphase have been deposited at different stages of constant pressure collapse, on hydrophilic Si(001) using a modified version of the inverse Langmuir-Schaefer method of horizontal deposition. The EDPs along the depth of the deposited films, extracted from the x-ray reflectivity data, show that a monolayer to bi-molecular layer transformation takes place after collapse. The molecules in the lower monolayer have asymmetric configurations with headgroups touching water and tails in air, whereas molecules in the upper layer are in symmetric configurations with tails on both sides of the heads. AFM images of the deposited films after collapse, however, show nearly circular islands of height more than that of the bimolecular layer observed in the EDP. As pressure increases, ridges are seen to coexist with these islands. Although the coverage of such islands and ridges is low, they play an important role in determining the growth mode. The growth of the wetting and island layers, taken together, has a striking similarity with the Stranski-Krastanow mode, observed usually for heteroepitaxial growth [published in Phys. Rev. E 73, 051608 (2006)].
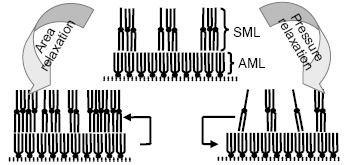 Langmuir monolayer is a fascinating example of 2-dimensional system, which is however, thermodynamically metastable. The study of relaxation, combining macroscopic and microscopic
information, can elucidate the processes involved in such system. Accordingly, the pressure relaxation (i.e. p-t curves for constant area) and the area relaxation (i.e. A-t curves for constant pressure) of preformed fatty acid salt, namely ferric stearate have been monitored. The presence of three distinct relaxation time is clear from the analysis of the relaxation curves. XRR analysis of the transfer monolayer on the other hand provides the processes those are involved. First two relaxation of short time scale (a few minutes and tens of minutes) can be associated with the reorganization of molecule in Langmuir monolayers, while the last one (nearly thousands of minutes) is due to the global material loss or desorption. It seems that the molecules move from the lower molecular layer to the upper molecular layer by changing 'asymmetric' to 'symmetric' molecular configuration under area relaxation. While in the pressure relaxation, the reverse process takes place, i.e., the molecules from the upper molecular layer come down to the lower molecular layer by changing their molecular configuration [published in Langmuir 24, 9386 (2008)].
Langmuir monolayer is a fascinating example of 2-dimensional system, which is however, thermodynamically metastable. The study of relaxation, combining macroscopic and microscopic
information, can elucidate the processes involved in such system. Accordingly, the pressure relaxation (i.e. p-t curves for constant area) and the area relaxation (i.e. A-t curves for constant pressure) of preformed fatty acid salt, namely ferric stearate have been monitored. The presence of three distinct relaxation time is clear from the analysis of the relaxation curves. XRR analysis of the transfer monolayer on the other hand provides the processes those are involved. First two relaxation of short time scale (a few minutes and tens of minutes) can be associated with the reorganization of molecule in Langmuir monolayers, while the last one (nearly thousands of minutes) is due to the global material loss or desorption. It seems that the molecules move from the lower molecular layer to the upper molecular layer by changing 'asymmetric' to 'symmetric' molecular configuration under area relaxation. While in the pressure relaxation, the reverse process takes place, i.e., the molecules from the upper molecular layer come down to the lower molecular layer by changing their molecular configuration [published in Langmuir 24, 9386 (2008)].
|

